Created in 2008, the Innovative therapies in myeloid leukemias and hematopoietic stem cells team deciphers the molecular and cellular mechanisms involved in the physiological and pathological differentiation of hematopoietic stem cells with a primary focus on cells of the myeloid lineage. We wish to propose new therapeutic opportunities for incurable myeloid hemopathies such as MDS, AML and CMML. Recent advances in the genetic diversity of MDS, AML and CMML have led to the development of more effective therapies, but their integration into a standardized clinical approach is still under debate. In order to contribute to the development and validation of innovative therapies for these hematological diseases, we need to better understand the molecular mechanisms involved in the transformation of myeloid cells. In this context, our team studies the regulation of apoptosis, autophagy, ferroptosis, oxidative stress, differentiation and reprogramming of myeloid cells. Our projects include both fundamental and applied research, with the objective of transferring our discoveries from the laboratory to the clinic through the development of innovative therapies for the benefit of patients.

Projects

G. RobertResearcher
Mail guillaume.robert@univ-cotedazur.fr

P. AubergerResearch Director
Mail patrick.auberger@univ-cotedazur.fr

The lysosome is a key organelle for the uptake and degradation of proteins through a spectrum of processes such as Macroautophagy and Chaperone-mediated Autophagy (CMA). In our research we have highlighted the importance of this organelle during aging and its importance in the genesis of myeloid leukemias. We are currently studying the impact of the invalidation of a lysosomal protein on the deregulation of the hematopoietic compartment and the genesis of myeloid leukemias, using original mouse models.
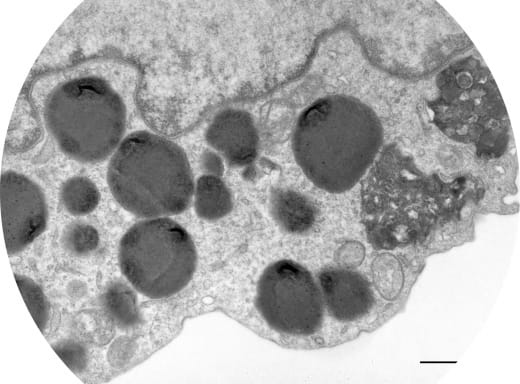
In collaboration with the Nice Institute of Chemistry, we are developing chimeric molecules called "biodegraders" capable of targeting oncogenic proteins involved in leukemia and dealing with them within the lysosome.

C. FavreauResearch engineer
Mail cecile.favreau@univ-cotedazur.fr

C. SavyResearch engineer
Mail coline.savy@univ-cotedazur.fr

M. BourgoinResearch engineer
Mail maxence.bourgoin@univ-cotedazur.fr

a. RivaultPhD student
Mail adele.rivault@etu.univ-cotedazur.fr

A. JacquelResearch Director
Mail arnaud.jacquel@univ-cotedazur.fr

P. AubergerResearch Director
Mail patrick.auberger@univ-cotedazur.fr

Cellular Reprogramming
My group focus on the study of myeloid immunosuppressive cells, such as anti-inflammatory macrophages (Leukemia-Associated Macrophages and TAMs) and MDSCs (Myeloid-derived suppressor cells), in hematopoietic malignancies (AML, MDS and CMML). The main function of these cells in cancer is to inhibit the antitumor immune response. Therefore, understanding the molecular mechanisms involved in the generation of LAMs and MDSCs might provide an opportunity for the design of novel therapeutic strategies. A better characterization of their origin, phenotype and ability to trigger an antitumor response and how these different myeloid immunosuppressive subpopulations could be impacted by anticancer therapies is urgently needed to selectively target/reprogram them in different types of leukemia.

E. KerreneurPhD student
Mail emeline.kerreneur@etu.univ-cotedazur.fr

M. BourgoinResearch engineer
Mail maxence.bourgoin@univ-cotedazur.fr

C. DelabyPhD student
Mail chloe.delaby@etu.univ-cotedazur.fr

A. AouadResearch engineer
Mail amaury.aouad@univ-cotedazur.fr

M. FajollesResearch engineer
Mail morgane.fajolles@univ-cotedazur.fr
Publications
Focus
Dual Covalent Inhibition of PKM and IMPDH Targets Metabolism in Cutaneous Metastatic Melanoma.Authors Zerhouni M, Martin AR, Furstoss N, Gutierrez VS, Jaune E, Tekaya N, Beranger GE, Abbe P, Regazzetti C, Amdouni H, Driowya M, Dubreuil P, Luciano F, Jacquel A, Tulic MK, Cluzeau T, O'Hara BP, Ben-Sahra I, Passeron T, Benhida R, Robert G, Auberger P, Rocchi S
Cancer research Jun 2021
IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential.Authors Boulakirba S, Pfeifer A, Mhaidly R, Obba S, Goulard M, Schmitt T, Chaintreuil P, Calleja A, Furstoss N, Orange F, Lacas-Gervais S, Boyer L, Marchetti S, Verhoeyen E, Luciano F, Robert G, Auberger P, Jacquel A
Scientific reports Jan 2018
Other recent publications
RAC2 gain-of-function variants causing inborn error of immunity drive NLRP3 inflammasome activation.Authors Doye A, Chaintreuil P, Lagresle-Peyrou C, Batistic L, Marion V, Munro P, Loubatier C, Chirara R, Sorel N, Bessot B, Bronnec P, Contenti J, Courjon J, Giordanengo V, Jacquel A, Barbry P, Couralet M, Aladjidi N, Fischer A, Cavazzana M, Mallebranche C, Visvikis O, Kracker S, Moshous D, Verhoeyen E, Boyer L
The Journal of experimental medicine Aug 2024
Emerging role of glutathione peroxidase 4 in myeloid cell lineage development and acute myeloid leukemia.Authors Auberger P, Favreau C, Savy C, Jacquel A, Robert G
Cellular & molecular biology letters Jul 2024
HSPA8 chaperone complex drives chaperone-mediated autophagy regulation in acute promyelocytic leukemia cell differentiation.Authors Rafiq S, Mungure I, Banz Y, Niklaus NJ, Kaufmann T, Müller S, Jacquel A, Robert G, Auberger P, Torbett BE, Muller S, Tschan MP, Humbert M
Pharmacology Apr 2024
Show all publicationsPatents
Co-inventors A. Jacquel, P. Auberger, M. Loschi, G. Robert, M. Bourgoin, M. Fajolles
Co-inventors O. Dufies, P. Chaintreuil, J. Courjon, A. Jacquel, P. Auberger, L. Boyer
Co-inventors L. Boyer, P. Auberger, J. Courjon, C. Pomares, V. Giordanengo, O. Visvikis, C. Loubatier, O. Dufies, C. Torre, A. Doye, P. Munro, R. Lotte, A. Jacquel, A. Robert, Stoyan Ivanov; Sebastien Vitale;


